Rules For Assigning Oxidation Numbers
Oxidation numbers are assigned to elements using these rules:
-
Dominion 1: The oxidation number of an element in its gratuitous (uncombined) country is zero — for example, Al(southward) or Zn(due south). This is too true for elements found in nature as diatomic (two-atom) elements
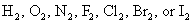
and for sulfur, found as:
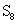
-
Dominion ii: The oxidation number of a monatomic (ane-cantlet) ion is the same equally the charge on the ion, for case:
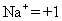
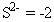
-
Rule 3: The sum of all oxidation numbers in a neutral compound is cypher. The sum of all oxidation numbers in a polyatomic (many-atom) ion is equal to the charge on the ion. This rule ofttimes allows chemists to summate the oxidation number of an cantlet that may take multiple oxidation states, if the other atoms in the ion take known oxidation numbers.
-
Rule iv: The oxidation number of an alkali metal (IA family unit) in a compound is +1; the oxidation number of an alkaline world metal (IIA family) in a chemical compound is +2.
-
Rule 5: The oxidation number of oxygen in a compound is usually –2. If, however, the oxygen is in a class of compounds called peroxides (for example, hydrogen peroxide), then the oxygen has an oxidation number of –1. If the oxygen is bonded to fluorine, the number is +1.
-
Dominion half dozen: The oxidation land of hydrogen in a compound is usually +1. If the hydrogen is part of a binary metallic hydride (compound of hydrogen and some metallic), so the oxidation country of hydrogen is –1.
-
Dominion 7: The oxidation number of fluorine is always –ane. Chlorine, bromine, and iodine normally accept an oxidation number of –1, unless they're in combination with an oxygen or fluorine.
![]()
Notice that the zinc metallic (the reactant) has an oxidation number of goose egg (rule 1), and the zinc cation (the product) has an oxidation number of +ii (rule 2). In general, you can say that a substance is oxidized when there'due south an increase in its oxidation number.
Reduction works the same way. Consider this reaction:
![]()
The copper is going from an oxidation number of +2 to zero. A substance is reduced if there'southward a decrease in its oxidation number.
About This Article
This article tin can exist constitute in the category:
- Chemistry ,
Rules For Assigning Oxidation Numbers,
Source: https://www.dummies.com/article/academics-the-arts/science/chemistry/rules-for-assigning-oxidation-numbers-to-elements-194221/
Posted by: easterdaytandon55.blogspot.com


0 Response to "Rules For Assigning Oxidation Numbers"
Post a Comment