What Does Negative Enthalpy Mean
What is Enthalpy?
Enthalpy is the measurement of energy in a thermodynamic system. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system'south internal energy plus the product of volume and pressure.
For more content on thermodynamics click here.
Technically, enthalpy describes the internal free energy that is required to generate a system and the amount of free energy that is required to make room for it past establishing its pressure and book and displacing its environs.
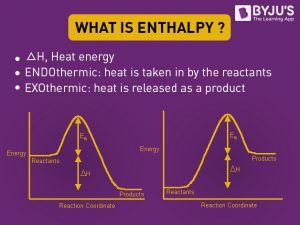
Enthalpy diagram
When a procedure begins at constant force per unit area, the evolved rut (either absorbed or released) equals the change in enthalpy. Enthalpy change is the sum of internal energy denoted by U and production of volume and Pressure, denoted past PV, expressed in the following style.
H=U+PV
Enthalpy is too described as a state function completely based on state functions P, T and U. Information technology is normally shown by the change in enthalpy (ΔH) of a process between the beginning and last states.
ΔH=ΔU+ΔPV
If the pressure and temperature don't change throughout the process and the job is limited to pressure and volume, the modify in enthalpy is given by,
ΔH=ΔU+PΔV
The flow of heat (q) at constant force per unit area in a procedure equals the change in enthalpy based on the following equation,
ΔH=q
A relationship betwixt q and ΔH can be defined knowing whether q is endothermic or exothermic. An endothermic reaction is the i that absorbs rut and reveals that heat is consumed in the reaction from the surroundings, hence q>0 (positive). If q is positive, so ΔH is too positive, at constant force per unit area and temperature for the to a higher place equation. Similarly, if the heat is released existence an exothermic reaction, the heat is given to the environment. Hence, q<0 (negative). Therefore, ΔH volition be negative if q is negative.
Also, Read: Enthalpy of Neutralization
Recommended Videos
Standard Enthalpy of Formation

Frequently Asked Questions – FAQs
Why is enthalpy useful?
Enthalpy is important because it informs u.s.a. how much oestrus is in a system (free energy). Heat is of import, since from information technology, nosotros can derive valuable piece of work. An enthalpy shift shows us how much enthalpy was lost or obtained in terms of a chemic reaction, enthalpy significant the system's heat energy.
What is the deviation betwixt enthalpy and internal energy?
The cumulative free energy that is stored in the device is internal energy. It is the amount held by the mechanism of potential and kinetic energy. Enthalpy is specified as the amount of the system's internal free energy plus the combination of the arrangement's gas force per unit area and its length.
What happens when enthalpy is positive?
A heat absorption reaction is endothermic. Its enthalpy volition exist positive, and its environment will cool downwards. This reaction (negative enthalpy, heat release) is exothermic. When the reaction happens, due to the gain in estrus the device emits, the atmosphere may rise in temperature.
What is enthalpy of chemic reaction?
The bonds betwixt atoms can dissolve, reform or both during chemic reactions to either absorb or release energy. The heat absorbed or emitted under constant pressure level from a device is referred to as enthalpy, and the reaction enthalpy is the change in enthalpy arising from a chemical reaction.
Is enthalpy always greater than internal energy?
The heat energy produced is often used in thermodynamics to improve the system'southward free energy or to practise some useful work. The energy associated with an open system is called enthalpy, which is often greater than or equal to internal energy.
To know more about a difference between enthalpy and entropy along with examples enthalpy equation and real-time examples at BYJU'S.
What Does Negative Enthalpy Mean,
Source: https://byjus.com/chemistry/what-is-enthalpy/
Posted by: easterdaytandon55.blogspot.com


0 Response to "What Does Negative Enthalpy Mean"
Post a Comment